Project 8A
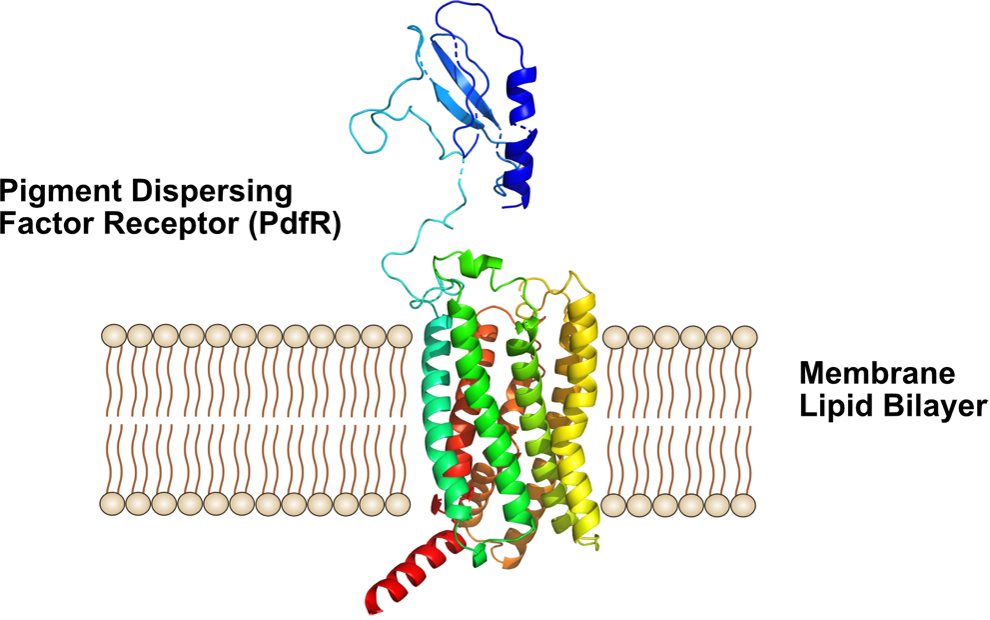
P8A: Biophysics of the Pigment dispersing factor receptor (PdfR)
Collaborators
Project description
Pigment dispersing hormone (Pdh) and pigment dispersing factor (Pdf) neuropeptides are required for the day-night rhythms, e. g. in insects. Studies in insects revealed that pigment dispersing factors (Pdfs), are coupling factors of circadian pacemaker cells, controlling rest-activity rhythms. Pdf is associated with the visual system and its expression levels are usually related to the changing light conditions in the environment. For signal transduction across membranes, Pdf binds to the pigment dispersing factor receptor (PdfR). How the PdfR transduces signals across membranes is not well understood. PdfR binds Pdf peptides in its extracelluar Pdf binding domain, which is thought to trigger structural changes of PdfR leading to transduction of the signal to G-proteins of the cytosol.
Approach and Methods
We recently established an Escherichia coli based over-expression system for isolation of the PdfR in milligramm amounts to examine its biophysics. For biophysical studies, we also developed protocols for solubilizing PdfR in detergent micelles and for the insertion of PdfR into lipid nanodiscs, which serve as model membranes. In this project, we will examine the stability of PdfR and the binding of Pdf using a range of biophysical methods. A focus will be on the site-directed spectroscopic labeling of the PdfR to confirm the predicted structure, the binding site, and to examine structure-function relationships.